A few days ago, my beloved fiancé surprised me with a GEOMAG construction toy for my birthday. GEOMAG consists of small rods with embedded magnets and metallic spheres that can be assembled into various structures. It’s truly amazing—I had no idea something like this existed!
This toy can effectively model crystallography problems. For example, below is a geometric proof of the Crystallographic restriction theorem
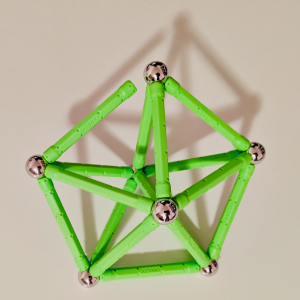
It can also demonstrate structural rigidity problems, as shown in this framework
which becomes flexible after removing just one link.
The stable framework is actually the snub trihexagonal tiling – a regular tessellation associated with the p6 packing of discs.
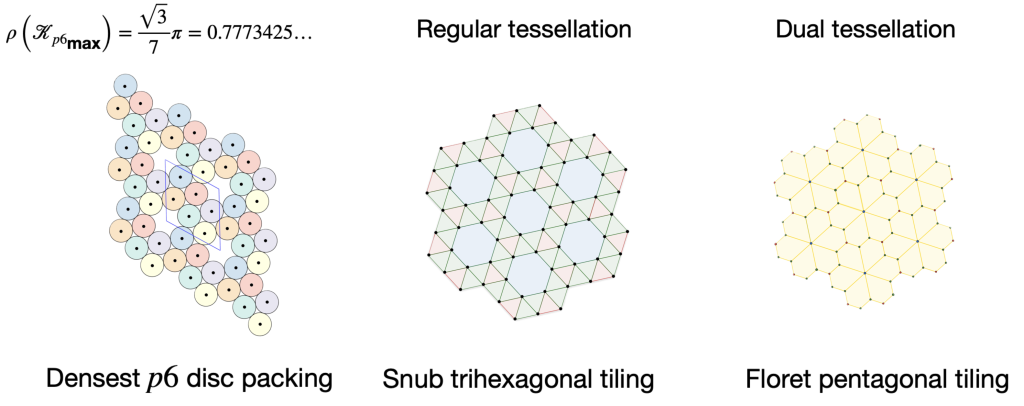
This framework is created by drawing links between the centers of touching disks. The tessellation on the right is called a floret pentagonal tiling. We can create this tiling from the p6 packing of hexagons by connecting each hexagon’s edge vertices to their nearest lattice points.
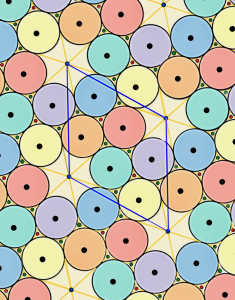
From the crystallographic perspective, the vertices of the dual tessellation lie in the
interstitial sites of the regular tessellation. Thus, the circles of the p6 packing are incircles of the pentagons in the floret pentagonal tiling and the hexagons in the hexagonal p6 packing.
This serves as a physical model of the Lennard-Jones system I described in my blog entry Lennard-Jones hexagonal molecular system. Through this model, I discovered that there are two chiral ground states connected by a continuous path in the configuration space.
Take a look at the animation below to see how it all works!
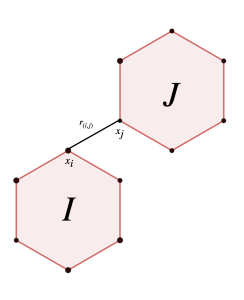
This is closely related to the vibrational part of lattice energy, degrees of freedom and structural stability of molecular crystals. Let’s have closer looks at this. Below is a visualization illustrating the transition between the chiral ground states of a 3-molecule cluster. Each molecule in the cluster contains six atoms. The intermolecular energy is given by the Lennard-Jones potential:
![Rendered by QuickLaTeX.com \begin{equation*} E_{LJ}=\sum_{\substack{\{I,J\} \\ I \cap J =0}} \sum_{\substack{(i,j ) \\ i \in I, j \in J}} \left[\left(\frac{1}{r_{(i,j)}}\right)^{12} - \left(\frac{1}{r_{(i,j)}}\right)^{6}\right] \end{equation*}](https://milotorda.net/wp-content/ql-cache/quicklatex.com-562681f5736399b7414a50ca69c95723_l3.png)
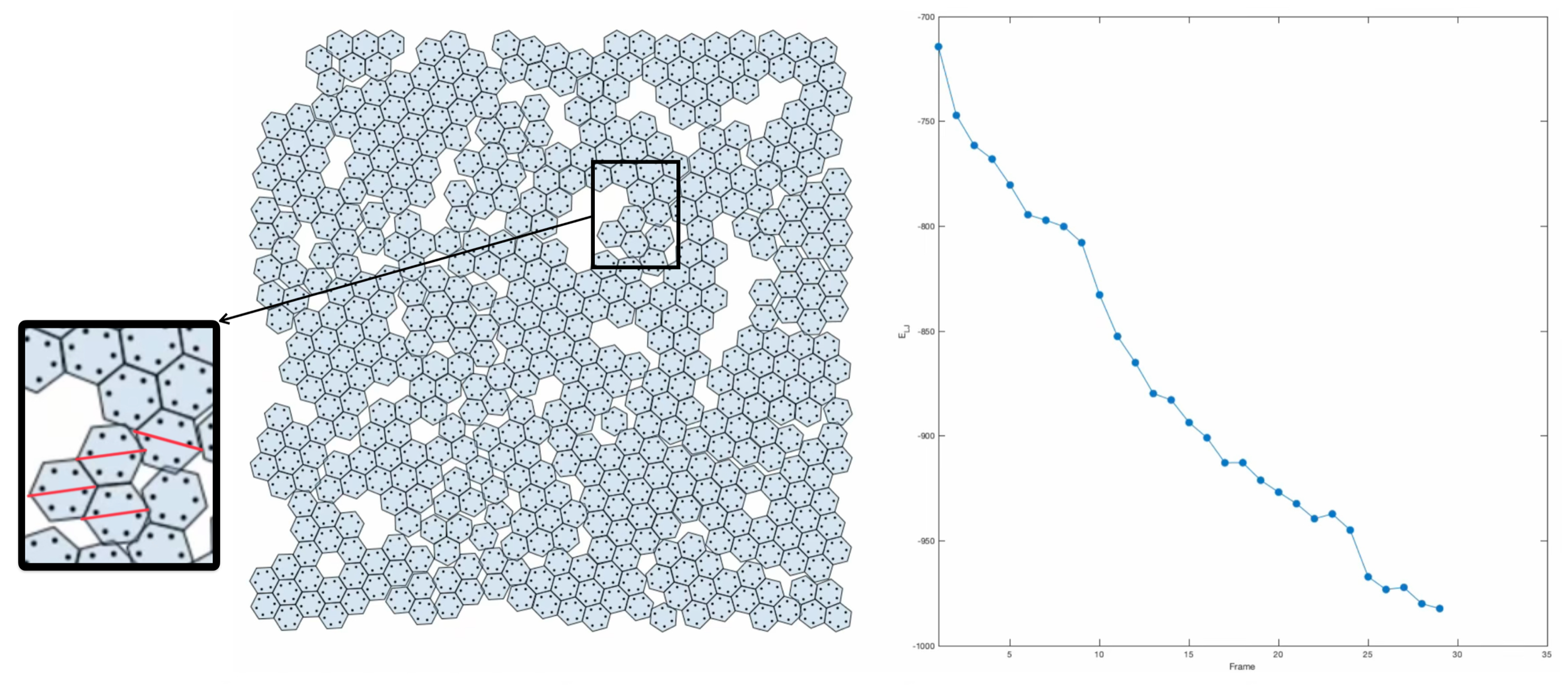
The chiral ground states are in clear energy potential wells and represent two different global solutions of the energy cluster ![]() minimization problem.
minimization problem.
From the GEOMAG model animation, one can imagine this cluster being held together only by the forces between nearest neighbors. By relaxing three of the total interactions, one introduces a single degree of freedom into the otherwise rigid cluster, allowing the configuration to shift into its neighbouring potential well.
However, this requires an interaction potential that decays to zero sufficiently quickly as the distance approaches infinity, since pairwise interactions among all atoms of different molecules are included, not only the first neighbours. The Lennard-Jones potential is one such example, where the leading contribution to the overall energy comes from the three-atom cluster in the middle, and the transition from one ground state to the other is animated as revolving around the centre of mass of this cluster.
It is also by this Lennard-Jones potential property that the ground state of this two-dimensional molecular system coincides with the densest ![]() packing of discs. This means that there are also two chiral densest
packing of discs. This means that there are also two chiral densest ![]() packings of all
packings of all ![]() -gons with six-fold rotational symmetry. This observation connects directly to my earlier work on plane group packings during my PhD, as it shows an aspect these packings I overlooked. The results of this study are published in our Densest plane group packings of regular polygons manuscript.
-gons with six-fold rotational symmetry. This observation connects directly to my earlier work on plane group packings during my PhD, as it shows an aspect these packings I overlooked. The results of this study are published in our Densest plane group packings of regular polygons manuscript.
Moreover, this explains the crystal defects in the Lennard-Jones hexagonal molecular system. Since mirror symmetry is not permitted in this two-dimensional system, we observe incompatible local ground state patches.
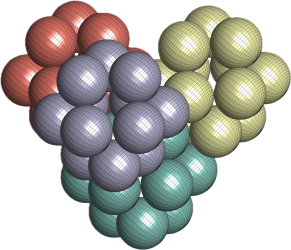
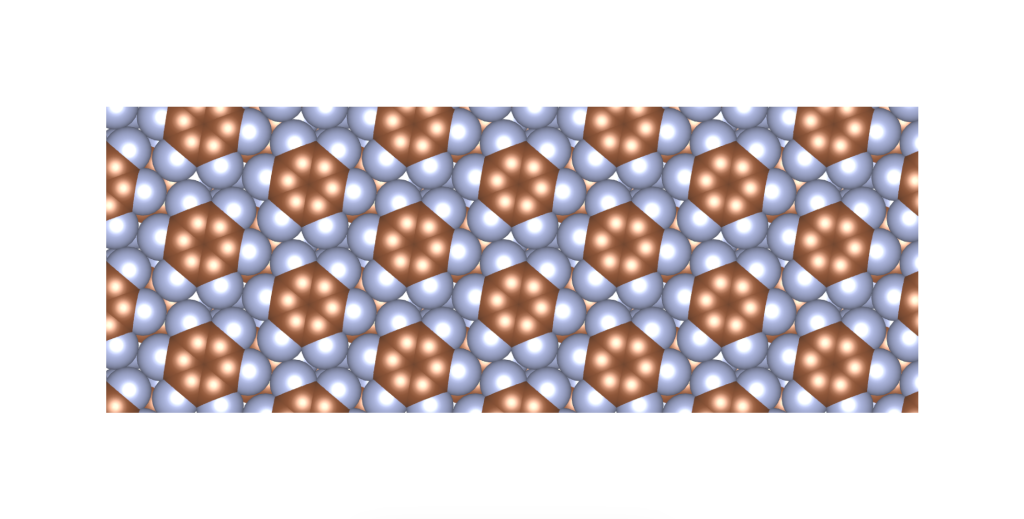
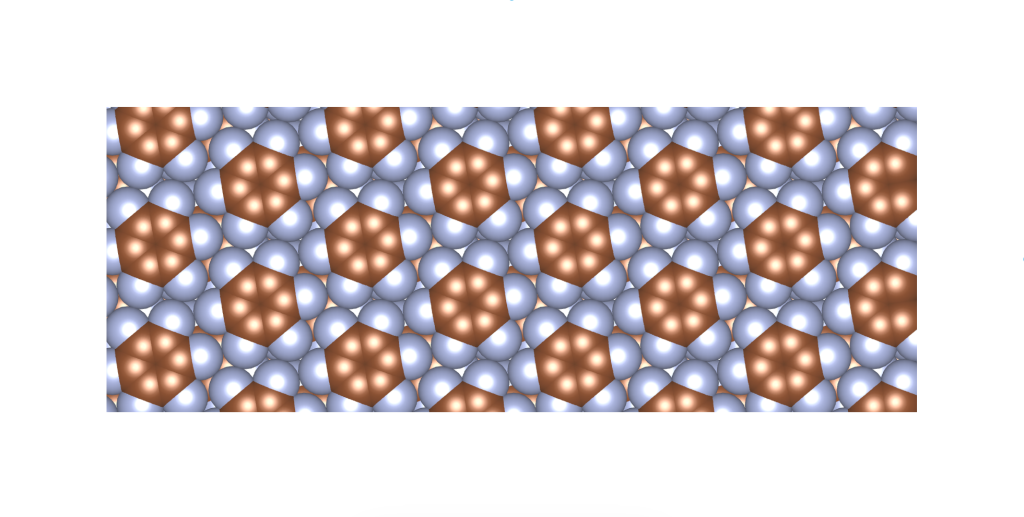
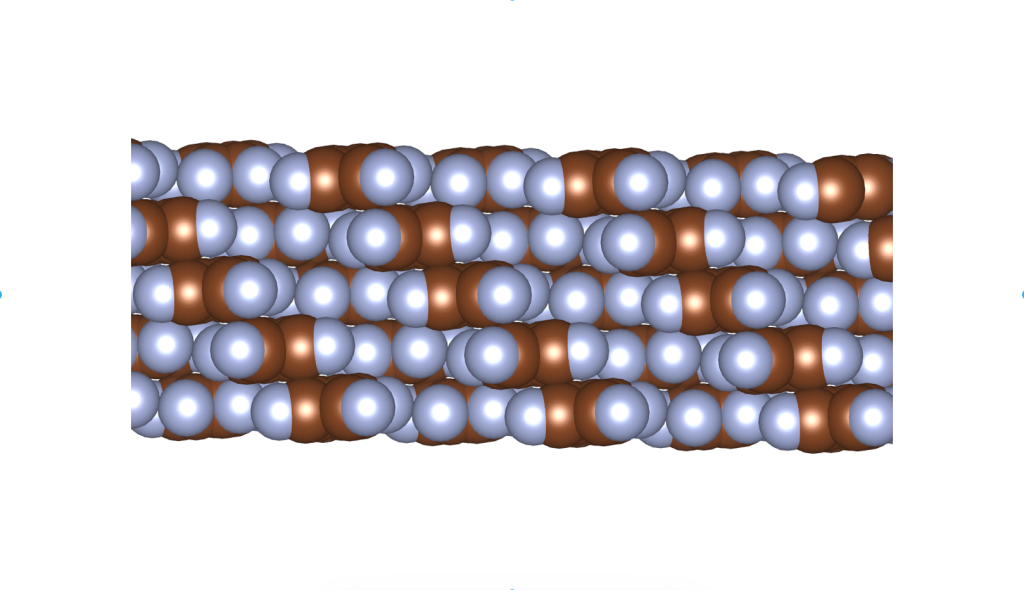
I was wondering if a real material with this crystal structure exists. In fact, it does, and it was published only a few years ago. See the image and reference below.
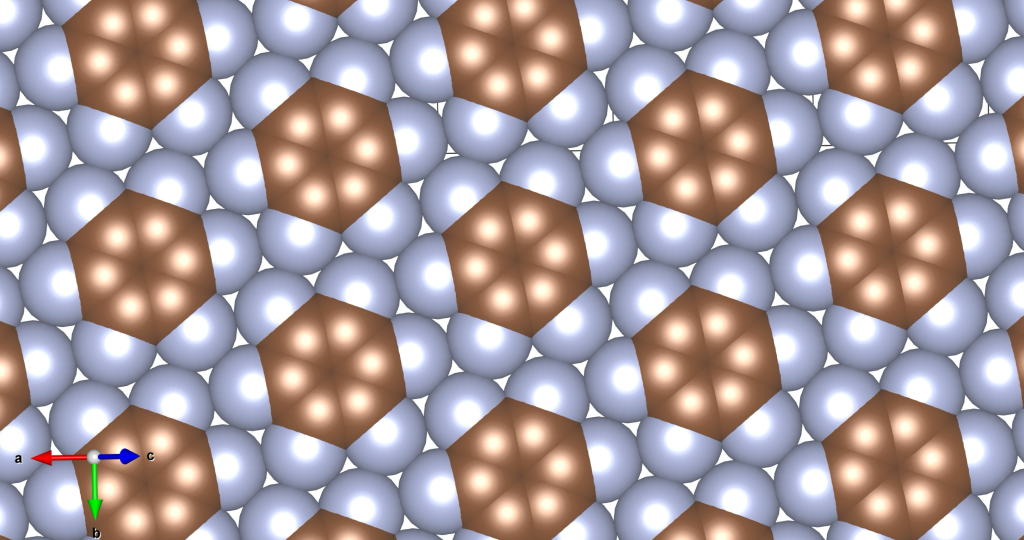
Rusek, M., Kwaśna, K., Budzianowski, A., & Katrusiak, A. (2019). Fluorine··· fluorine interactions in a high-pressure layered phase of perfluorobenzene. The Journal of Physical Chemistry C, 124(1), 99-106.
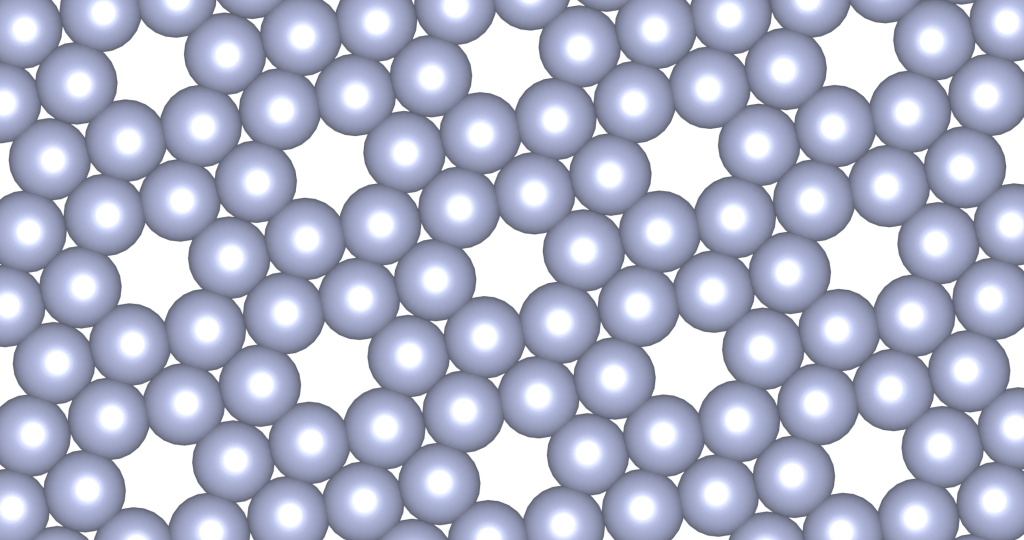
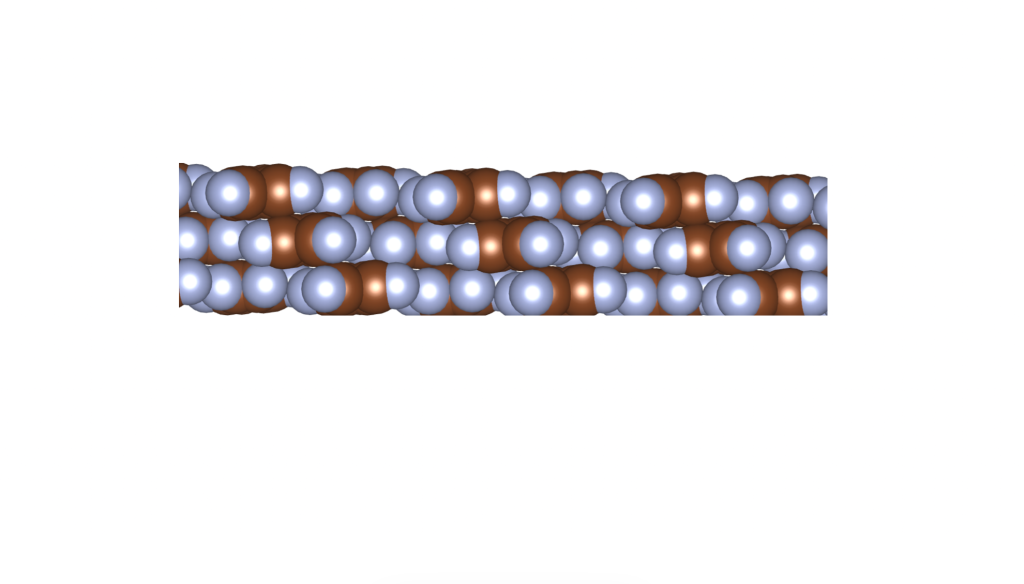
The above is a space-filling visualization of this high-pressure phase of perfluorobenzene, which is in excellent agreement with our highly simplified model. The sphere radii are set to the van der Waals radii. The left image shows one layer, while the right image displays the same layer with the carbons removed. The space group of this crystal is C2/c. C2/c symmetry consists of a two-fold rotational symmetry (here, within one layer of perfluorobenzene) and a perpendicular mirror symmetry. This effectively means that the layers alternate as two chiral two-dimensional ground states, interchanging chirality layer by layer.
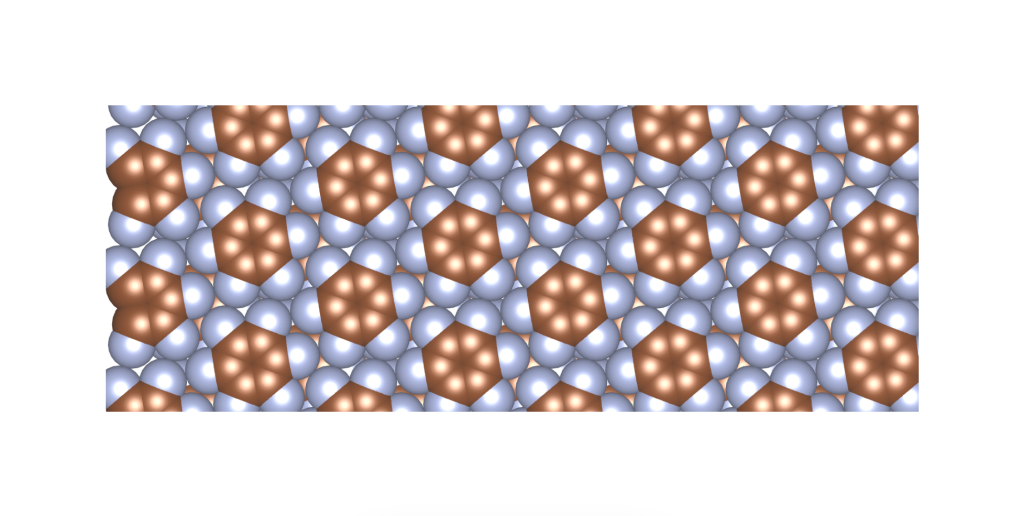
S – enantiomer
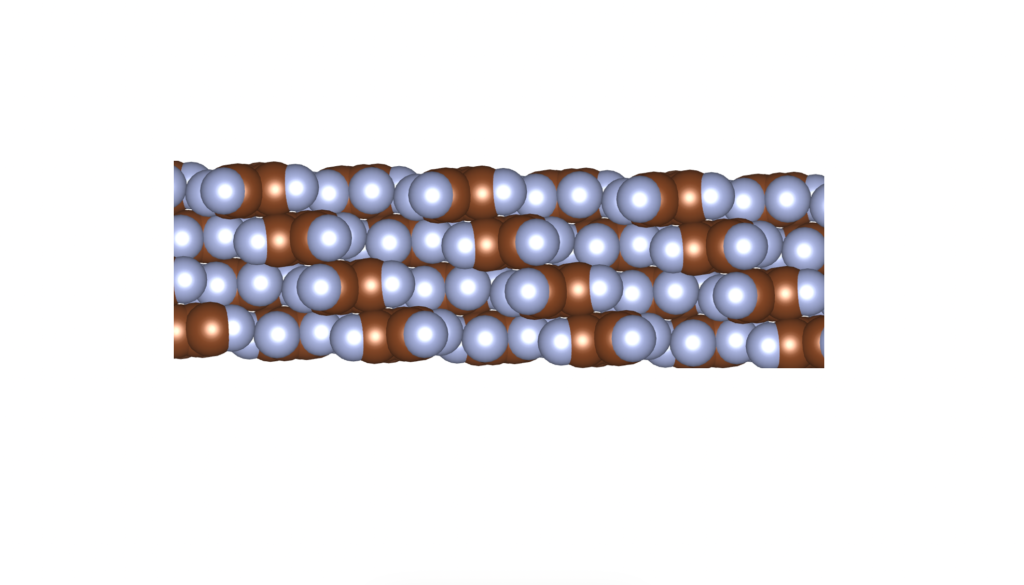
R – enantiomer
S – enantiomer
Why is this important beyond just creating appealing pictures and animations? One reason is that it can significantly reduce computational time complexity by eliminating unnecessary calculations in
Crystal Structure Prediction (CSP). For example, if we know beforehand that our target compound satisfies a few assumptions, the degrees of freedom to explore can be reduced to a maximum of 12, sometimes even less, as demonstrated in our Close-Packing approach to CSP (Alternatively, we can think of the hard sphere model as a pairwise interaction potential. However, in this blog, we are considering soft core interactions).
And we know time is money, as illustrated by the recent developments around the DeepSeek model and its ensemble learning approach to large language model training. The concept of ensemble here mirrors the
Thermodynamic ensemble that Gibbs introduced in 1902.
This also shows how chemistry can inspire the development of novel machine learning algorithms beyond Machine-learned interatomic potentials.